State News
Richmond Circuit Court upholds Executive Order Forty-Nine
RICHMOND—The Richmond Circuit Court today upheld Governor Ralph Northam’s decision, outlined in Executive Order Forty-Nine, to temporarily ban firearms on Capitol grounds from 5:00 PM on Friday, January 17, 2020 until 5:00 PM on Tuesday, January 21, 2020. The Governor said that law enforcement intelligence analysts had identified credible threats of violence surrounding the event, along with white nationalist rhetoric and plans by out-of-state militia groups to attend.
Governor Northam issued the following statement:
This is the right decision. I took this action to protect Virginians from credible threats of violence. These threats are real—as evidenced by reports of neo-Nazis arrested this morning after discussing plans to head to Richmond with firearms.
I’m grateful to the Circuit Court for recognizing the seriousness of these threats, and for upholding this reasonable, legal action to protect all Virginians, including demonstrators and policymakers. I will continue to do everything in my power to keep Virginians safe.
Agriculture
Feds to Require Bird Flu Tests of Dairy Cattle Before Transport
Starting next week, certain dairy cattle must be tested for avian influenza before they can be transported to a different state, the U.S. Department of Agriculture announced Wednesday.
The requirement is among several that will expand the testing, reporting, and monitoring of the cattle to reduce the spread of bird flu among the animals.
The new rules follow evidence that highly pathogenic avian influenza — which is commonly spread by migrating birds — has transmitted from cow to cow and from cattle to poultry, and that infected cows might not show symptoms of illness, the USDA said. Last week, an analysis of the virus from a Kansas cow showed that it had acquired “an adaptation to mammals.”


USDA will require certain dairy cattle to be tested for avian influenza before they can be transported to a different state. (Photo by Scott Bauer/USDA Agricultural Research Service)
On Tuesday, the U.S. Food and Drug Administration said tests had revealed fragments of the virus in pasteurized milk, but that they don’t pose a risk to public health.
“While we are taking this action today, it is important to remember that thus far, we have not found changes to the virus that would make it more transmissible to humans and between people,” the USDA said Wednesday.
It has been a month since the virus was first confirmed to have infected dairy cattle in Texas. The virus has now been detected in 33 dairy herds in eight states, the USDA said. Part of that spread has been attributed to the transportation of infected cows to new herds.
The cows most often recover from infection after a week or so, but their tainted milk cannot be used for commercial human consumption. The virus is often deadly for poultry and can rapidly infect flocks.
The rules set to take effect on Monday require lactating dairy cattle to test negative for influenza A before they are transported across state lines, and that requirement might be expanded to other types of dairy cattle in the future.
Labs must also report their confirmed infections of livestock to the USDA, and certain herd owners must provide details about where their cattle have been transported.
Further information about the new rules is forthcoming, and state agriculture officials declined for now to say what impact they will have on Iowa dairy farmers.
“We are still awaiting specific guidance from USDA regarding this new interstate movement order,” said Don McDowell, a spokesperson for the Iowa Department of Agriculture and Land Stewardship.
John Maxwell, a dairy farmer near Davenport, Iowa, predicted that the effects for most dairies in Iowa will be inconsequential and that it’s best to be cautious and increase testing until more is known about the disease.
“We have to do tests anyway,” he said, in reference to dairy cattle he sells out-of-state. “So it would be one more test and whatever the cost it might be. One more is not the end of the world.”
The USDA has said it will reimburse farmers for testing of sick and asymptomatic cattle.
States with confirmed bird flu infections of dairy cattle include Kansas, Idaho, Michigan, New Mexico, North Carolina, Ohio, South Dakota and Texas.
A virus similar to what has infected cows has been found in poultry flocks in Kansas, Michigan, Minnesota, New Mexico and Texas, the USDA said.
by Jared Strong, Virginia Mercury
Virginia Mercury is part of States Newsroom, a nonprofit news network supported by grants and a coalition of donors as a 501c(3) public charity. Virginia Mercury maintains editorial independence. Contact Editor Samantha Willis for questions: info@virginiamercury.com. Follow Virginia Mercury on Facebook and Twitter.
State News
Fires Have Consumed Nearly 20,000 Acres in Virginia This Spring. That Could be Good for the Environment.
Almost 20,000 acres have been lit by flames that primarily torched the western and central parts of the state so far during Virginia’s 2024 spring fire season. With about a week left until the season ends, that is double the amount of acres affected annually in the state across its 10-year average.
There’s no question that the fires visibly caused an immediate loss of vegetation and wildlife habitat, but state and federal officials said in interviews with the Mercury last week the blazes provide some benefits and are a centuries-old resource management tool.
“It does play an important role in the ecosystem,” said Michael Downey, assistant director for wildfire mitigation and prevention at the Virginia Department of Forestry. “In the public’s eye it is a natural disaster, but we do try to keep it in a controlled, contained environment.”
Prescribed, or controlled, blazes are regularly implemented by state and federal agencies, which include the Department of Forestry, the Department of Wildlife Resources and the U.S. Forest Service. It’s the unruly nature of the wildfires that can cause concern, particularly given the proximity to neighborhoods and communities where people live.
“We don’t want people thinking, ‘Let’s go start a wildfire,’ but there are benefits,” said Michael Puckett, a small game project leader at DWR, adding that the fires are not solely a matter of loss of wildlife habitat, but a “matter of change.”
It’s the human communities abutting the wooded areas that are inhibiting wildlife’s ability to roam freely to and from impacted areas. Humans also contribute to some of the causes of the fires.
“As wildfires grow in severity/intensity, we will see species moving in new patterns and places in order to find new habitat,” both immediately after fires and in the longer term as species’ ranges shift, said Misty Boos, U.S. conservation policy manager at Wildlands Network.
“This underscores the importance of protecting large, connected landscapes and wildlife corridors so species can move and adapt, but it also demonstrates the importance of wildlife coexistence.”
Flora and Fauna
Starting at the ground level, the fires’ effects can matriculate down into the soil, depending on the severity, determined by fire intensity and duration.
The fires’ effect can increase dirt’s water repellency, or inability to hold water, leading to it eroding and potentially ending up in waterways.
Following the fires that hit the state in 2016, researchers at Virginia Tech found that some severely-burned areas were water repellent at rates of 68-74%. The unburned areas showed water repellency at a rate of 0-18%, the research found.
“A lot of fires in [Virginia] don’t get as large or hot as those out west, but in local areas we can see pretty severe burn severities,” said Ryan D. Stewart, an associate professor at Virginia Tech.
“Areas that have moderate to severe burn severities can have issues like the upper duff and organic layers being consumed, and development of a layer a few inches deep that does not easily rewet.”


A forest in Highland County during a prescribed burn by the Virginia Department of Forestry in 2021. (Sarah Vogelsong/Virginia Mercury)
On the flora aspect, the clearing of taller trees can pave way for sunlight to reach the lower level vegetation, said Puckett. Creating a more diverse portfolio of vegetation within the forest can create a more diverse ecosystem, added Lane Gibbons, fire management specialist at Shenandoah National Park.
“If you kind of think of it in terms of investing, you don’t invest all of your money in one thing. That’s too much of a gamble,” said Gibbons. “You really want a diverse portfolio. It works very [similarly] in forests. If you have more of a diverse portinfo — tall versus short, young versus old — if you have a greater variation [and] then you have a greater variation of types of organisms using those resources.”
Over time, forests in Virginia have become more resilient, with thicker oak trees popping up in places more susceptible to fires, Gibbons added, with less-deterrent maple pines growing in areas less likely to catch a blaze.
While oaks may be stronger, they also can attract invasive animal species, like the Spongy Moth, whose presence requires some maintenance and can be found throughout the state.
The caterpillar-like creatures provide benefits to forested areas by thinning out trees, allowing other plants to grow. But the bugs feed primarily on the oaks attracting them, which, in addition to their fire resilience, provide numerous benefits to the climate, including capturing carbon in the atmosphere.
“We’re looking at ways to bring back oak and fire is one of those ways to do a timber stand improvement,” Downey said, describing the process of removing undesirable species and then setting fires to bring back nutrients into the soil. “That’s sometimes what oak needs for it to regenerate.”
On the fauna aspect, the Wildlife Center of Virginia took in a bear cub found to suffer from smoke inhalation. Smaller amphibious animals like the box turtle suffer from the havoc wreaked by the blazes, because they live in small brush or leaf litter and can’t move out fast enough.
But larger wildlife that call the western parts of the state home, like turkey or small game like squirrels, may be displaced immediately, but sometimes they can be seen returning to the area before the smoke clears, Puckett said.
“We have enough moisture in the system here,” said Puckett, adding that wildlife can return within a year. “It’s not like cases out west that may burn down into the soil with the dry climate and lack of rainfall. Things don’t tend to recover as quickly as they do here.”
Human influence
It’s often humans, who infringe on animal habitats, that create cause for concern related to wildfires.
According to information released in January by the Weldon Cooper Center for Population Estimates, some rural areas of Virginia saw losses in population while others saw gains. Page County’s population grew by 2 to 4% from 2020 to 2023. Some central and eastern areas of the state, including Louisa County, grew by over 4%.
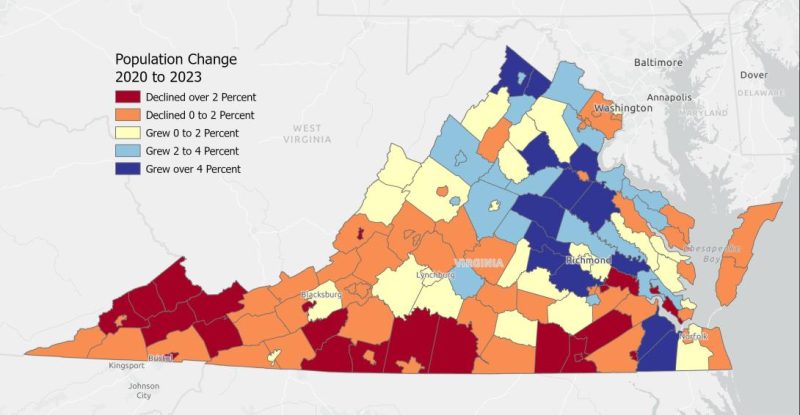

Population change from 2020 to 2023. (Courtesy of Weldon Cooper Center Population Estimates)
Those increasing populations spur the development of communities abutting wooded areas that frequently prevent wildlife from being able to roam freely away from fires. Republican Gov. Glenn Youngkin signed House Bill 309 and Senate Bill 461, which directs the Virginia Department of Forestry to create a plan that includes protection of wildlife corridors, and large contiguous blocks of forests.
“As we’ve seen, events like wildfire (as well as floods, hurricanes, extreme snow storms, etc.) can temporarily bring wildlife into closer proximity to people, which can cause conflicts,” said Boos, with Wildlands Network.
More development means more utility infrastructure, such as electric power lines, getting built. The strong winds this past season that led to power lines being knocked down and sparking blazes, instead of natural causes like lightning strikes that happen in Alaska.
“80 to 90% of fires are caused by humans,” Downey said.
When asked about downed power lines causing some of the fires this past spring, spokesperson for Shenandoah Valley Electric Cooperative said the utility, “will continue to cooperate with all affected localities to assess damage as we rebuild damaged power grid infrastructure.”
“This widespread event, combined with extremely low humidity, made conditions favorable for wildfires,” said Preston Knight, SVEC spokesperson. “Many communities throughout our service territory have experienced wildfires and our hearts go out to those who have suffered anguish and loss.”
Residents can clear debris from around their homes to prevent the fires from spreading, a task the Department of Forestry can help with despite their limited capacity, Downey said.
“We can only do what we can with our resources,” Downey said.
Impact going forward
Leading up to the fall and spring fire season, there were periods of drought identified by the Department of Environmental Quality. A report from the U.S. The Department of Agriculture found that “increased fuel load and more frequent droughts may increase wildfire frequency and intensity within the Southeast.”
That same USDA report said ways to make forests more resilient included, “taking steps necessary to appropriately manage stand density, hydrologic characteristics, and natural habitats,” and that these steps “can also have a positive impact on the ecological functioning and overall health of the forest.”
Adding fuel to the fire, literally: A study out of the University of California Riverside found plants are more easily burning as a result of absorbing more carbon that’s in the air, carbon created by pollution.
Creating markets for pulpwood and biomass that come from the over 16 million acres of forests in Virginia, about 80% of which are privately owned, can help reduce fuels by removing “less desirable species and residuals from the understory and floor of the forest,” said Corey Connors, executive director of the Virginia Forestry Association.
One of the authors of the University of California study’s said in a statement that, “we do need to implement better fire control and have more prescribed burns to use up plant fuel. We need to get rid of the old stuff.
“But the best way to decrease wildfires is to mitigate our carbon dioxide emissions,” Gomez said. “We need more emission control now.”
In Virginia the largest sources of emissions are transportation, followed by the commercial industry sector and electricity generation, according to DEQ.
While international research points to human-created emissions causing climate change, the impacts of climate change on the fires affecting the adaptability of the ecosystem in forests is still being determined, Gibbons said.
“It’s a topic that we’re trying to figure out,” he said. “We’ll implement strategies as we learn more.”
by Charlie Paullin, Virginia Mercury
Virginia Mercury is part of States Newsroom, a nonprofit news network supported by grants and a coalition of donors as a 501c(3) public charity. Virginia Mercury maintains editorial independence. Contact Editor Samantha Willis for questions: info@virginiamercury.com. Follow Virginia Mercury on Facebook and Twitter.
State News
Commuter Train System Eyes Expansion, Part of Virginia’s Evolving Rail Trends
While 2050 is more than a quarter century away, The Virginia Railway Express wants to start transforming its commuter rail operations much sooner by offering Saturday services as it considers its System Plan 2050, part of holistic, multi-agency efforts to transform rail services in the commonwealth.
Last year, the VRE Operations Board — which is represented by the nine jurisdictions that fund the commuter rail service — backed the agency’s budget that included a 5% fare hike, or 50 cents more, due to the increase in services since 2020. The budget also included a plan to, for the first time, operate Saturday train service on tracks shared with Amtrak, CSX and Norfolk Southern.
The next step is for the board to endorse the agency’s System Plan 2050, designed to help officials address the public’s changing travel patterns, including those of commuting office workers, which have shifted over the past decade. The board is scheduled to vote on the plan June 21.
The plan is built around four goals: safety and reliability; market growth and financial stability; regional system integration and equitable service; and sustainability and resiliency. The goals of market growth and equitable service focus on maximizing daily riders and expanding daily service offering non-peak and weekend service.
“We have four system plan goals for the 2050 plan and each goal is important. However I believe goal #2 is the most important because we must grow our market for VRE service while maintaining financial stability; provide service for a full range of travel given the changing needs of the regions,” said Meg Bohmke, chair of the VRE Operations Board.
Board member James Walkinshaw added that while the board is considering expanding service, it intends to continue meeting commuters’ demands as well.
The plan
Overall, VRE’s plan will accommodate commuters, but also families and travelers who want to tour Washington D.C., and historic areas and battlefields in Virginia through expanded Saturday services, and potentially more daily weekday trips and an express service.
VRE operates two rail lines originating from Washington D.C. The first rail line runs to Spotsylvania and the second to Broad Run in Prince William County.
By surveying passengers, officials learned that telework rates are higher than before the pandemic, and passengers believe it’s faster to drive than take public transit.
Respondents also asked for more schedule flexibility, better connections to VRE stations, and more support for non-commuters.
“I know a lot of folks who live in Richmond and work for the federal government, who utilize VRE, and they would certainly like to do it more with later trains, earlier trains, and maybe trains that don’t fit that normal pre-pandemic commuting pattern,” said Danny Plaugher, executive director of Virginians for High Speed Rail.
Plaugher said he’s also excited about the VRE’s emphasis on reducing environmental impacts.
“I think there’s going to be a tremendous opportunity for VRE in the future, and it is exciting that they’re beginning that process now for how they can continue to evolve on that front,” Plaugher said.
To meet the goals of the proposed plan, Walkinshaw said VRE’s plan would require additional locomotives, passenger railcars and personnel to upgrade its rail commuter services, which they’ve offered since 1992.
Walkinshaw said VRE already has the railroad tracks to make the 2050 plan work, but there are more components that will be needed, including funds to help increase the rail capacity of the Long Bridge from Washington D.C. to Virginia. In December, Virginia leaders announced the state received $729 million in federal funds to help pay for the expansion of the bridge that crosses over the Potomac River.
Further investments will be used to extend platforms, improve stations and add parking at key stations.
The past chair said the plan creates a transit system that he believes the region “needs and deserves.”
“VRE is going to be a key piece of the overall region’s transit network between now and 2050 in a much more substantial way than it is even now,” Walkinshaw said.
Transforming rail
Simultaneously, as VRE’s System Plan 2050 is being finalized, the Transforming Rail in Virginia program is enacting a more wide ranging effort to improve passenger and rail service along the Interstates 64, 85 and 95 corridors.
According to the Virginia Department of Transportation, the program would allow Virginia to double Amtrak’s state-supported service and increase VRE services over the next decade.
The program would also build the foundation for higher-speed passenger rail service to the Southeast by acquiring the abandoned S-Line, running from Petersburg into North Carolina.
Plaugher said having more rail options for travelers is essential, and VRE’s plan is also beneficial because it helps get more cars off the road as the leaders seek to reduce carbon emissions.
“Commuter rail is a huge component of transforming rail,” Plaugher said. “Having alternatives is very important, and so this proposal, I think, fits perfectly within the Transforming Rail [in Virginia].”
While he’s uncertain what future capacity will look like by 2050, Plaugher said it’s good that VRE has started to plan for the future.
“And one thing I’ve heard from our transit agencies, especially the commuter-based ones, is that even though the ridership is lower than pre-pandemic, more people are actually using the service overall.”
According to data from the Department of Rail and Public Transportation, VRE recorded 20,036 passengers in 2021. Ridership increased in the following two years: 37,487 passengers in 2022 and 120,228 in 2023.
This story was updated to add Bohmke’s remarks and to correct the terminal location of the VRE’s Broad Run line.
by Nathaniel Cline, Virginia Mercury
Virginia Mercury is part of States Newsroom, a nonprofit news network supported by grants and a coalition of donors as a 501c(3) public charity. Virginia Mercury maintains editorial independence. Contact Editor Samantha Willis for questions: info@virginiamercury.com. Follow Virginia Mercury on Facebook and Twitter.
State News
Virginia School Board Files $600K Lawsuit Against Father of Special Needs Student
The Bedford County School Board filed a lawsuit seeking $600,000 in damages from the father of a special needs student, claiming the man’s abrasive communications with school staff about his son’s treatment over the last three years amounts to illegal intimidation and harassment.
In court filings, Bedford resident David Rife insists he’s the one being intimidated, noting that the county school board sued him shortly after he filed a complaint with the Virginia Department of Education saying local school officials weren’t following the individualized education program, or IEP, designed to accommodate his son’s learning disability and improve his reading skills. When he filed the complaint, Rife told state officials he feared he would face retaliation locally, according to court documents.
The school board filed its lawsuit against Rife on March 26, roughly a month after Rife sent his complaint to state education officials.
In a March 29 letter responding to Rife’s complaint, state education officials ruled Bedford school officials were out of compliance in several policy areas and ordered the division to take corrective action in regards to Rife’s son by April 29.
In his answer to the lawsuit, Rife suggested the timeline “suggests the possibility” the suit may have been retaliation and argues it’s school officials who are in the wrong.
The court case highlights ongoing disputes over parents’ rights in K-12 schools, spotty compliance with special education rules and how public officials should handle unpleasant or hostile speech from critics.
Virginia adopts regulatory changes for special education amid federal review
The case was brought on behalf of the Bedford School Board by attorneys in the Richmond office of Sands Anderson, a law firm that handles a variety of local government issues. A Sands Anderson attorney didn’t respond to a request for comment on Rife’s claims about the motivations for the suit.
Bedford School Board Chairman Marcus Hill didn’t respond to requests for comment Monday. The school board itself is the plaintiff suing Rife.
In court filings responding to the suit, Rife and his attorney contend he had good reason to be frustrated with Bedford schools over the lack of consideration for state and federal special education laws. Rife’s defense contends he only used “impolite” language while “passionately advocating” for his son, who suffers from anxiety and was reading at a fifth grade level as a sophomore in high school.
“It is highly ironic that BCPS, whose School Board members have emphasized repeatedly and strongly the importance of protecting parental rights, have now sued a parent for a total of $600,000.00 because the school personnel did not like how he has advocated for his son,” said the response filed on Rife’s behalf by Bedford attorney David Whitehurst.
The civil suit claims Rife used “obscene, vulgar, profane, lewd, lascivious and/or indecent language” when interacting with school staff in person, over the phone and via email. The lawsuit lists several examples of Rife threatening to call the police, have people arrested, file lawsuits or initiate investigations over what he believed to be mistreatment of his son.
The filing recounts conversations in which Rife allegedly said the school principal should “get her head out of her ass” and said her “bullshit” was putting his son at risk. Documents filed in court also suggest Rife used the terms “asshole,” “jerk” and “jerkoff” to refer to school staff members.
Bedford school officials tried to put Rife on a communications plan in early 2023 that limited his contact with staff.
“Bedford County Public Schools expects parents to be respectful and civil when interacting with school staff members,” Deputy Superintendent Karen Woodford wrote to Rife last July in a letter filed with the lawsuit. “We will no longer tolerate your profanity to staff, threats, dishonest communication about people losing their jobs, yelling and speaking badly about staff members. If you cannot abide by this plan, I will limit your interaction with staff even more than it is at this time.”
In addition to the $600,000 the school board is seeking from Rife for “personal injury, lost profits, increased operating expenses and legal fees,” the board is asking the Bedford Circuit Court to order Rife to stop using vulgar language and follow the communications plan the school division laid out for him.
“Putting an end to the abuse of government resources is plainly in the public interest,” the school board’s lawyers wrote in a motion seeking an emergency injunction against Rife.
Rife’s attorney has argued that nothing he said rises to the level of criminal conduct under the laws the school board pointed to dealing with using a computer or phone for harassment and “abusive language to another.” The communication plan the school division is asking the court to enforce states that Rife can only use email to contact the school principal and the division’s director of special education and says they “will not communicate through phone unless there is an emergency.”
In a competing motion asking the court to order Bedford County Public Schools to comply with the IEP plan, Rife’s side argued the injunction school officials were seeking would infringe on free speech.
“It would serve as a ‘lesson’ to other parents that advocating for their children could result in being sued,” Rife’s motion says.
The communications the school divisions objected to, Rife’s attorney argued in legal filings, should be understood in the context of a parent trying to help their child but getting little assistance from school officials. Many of the interactions school officials spotlighted involved Rife complaining about bullying by other students or behavior by teachers that he felt was adding to his son’s difficulties at school, according to court records.
“One example is that Mr. Rife has been restricted from speaking directly with his son’s case manager or the reading specialist for over three years outside of IEP meetings,” Rife’s court filings say. “As would most parents in such a situation, this has caused him to become frustrated and upset, and that frustration has at times been expressed to school employees, but never with malice or threat.”
The specific language school staff found offensive would “generally be considered fairly mild in today’s culture,” Rife’s court filings argue.
State officials ruled Bedford officials were not in compliance with several sections of the student’s IEP calling for him to receive specialized instruction and assistance. For example, the plan called for the student to receive at least 45 minutes of reading services five times per week in a special education setting, but state officials determined he wasn’t always getting that level of instruction.
The state ordered Bedford school officials to reexamine the student’s IEP, discuss whether he needed extended school year services and review what “compensatory” assistance was necessary to address the impact on the student’s “access to a free appropriate public education.”
Attorneys for the school board portrayed the lawsuit as a necessary step to safeguard the school division after less formal efforts to improve relations with Rife failed.
The courts requiring Rife to communicate with school officials in a particular manner, the school board’s lawyers wrote, won’t impede his ability to have “reasonable, good faith communications with BCPS about his child’s education.”
A hearing has not yet been scheduled in the case.
by Graham Moomaw, Virginia Mercury
Virginia Mercury is part of States Newsroom, a nonprofit news network supported by grants and a coalition of donors as a 501c(3) public charity. Virginia Mercury maintains editorial independence. Contact Editor Samantha Willis for questions: info@virginiamercury.com. Follow Virginia Mercury on Facebook and Twitter.
State News
Youth Violence Prevention Program Funding Hangs in the Balance as Legislature Reworks State Budget
Two Virginia school divisions are slated to launch a pilot program intended to help reduce youth involvement in gangs and violent behaviors with guns but it’s unclear if the initiative will be fully funded, as lawmakers go back to the drawing board to work up a new state spending plan.
On April 2, Gov. Glenn Youngkin signed legislation to create the Community Builders Pilot Program that will start with Roanoke and Petersburg City Public Schools students entering the eighth grade. Pupils in both districts face high rates of gun violence and cases of students bringing firearms to school.
Bill carriers Sen. Lashrecse Aird, D-Petersburg, and Sam Rasoul, D-Roanoke, said unlike other community violence intervention efforts centered around getting weapons off the streets, their legislation takes a different approach because it centers students.
“We’re hoping by involving young people that perhaps it helps in other ways,” said Aird, adding that such a program could also have a “residual impact” on children facing disciplinary trouble in school.
“But ultimately, [this legislation] is specifically trying to make sure that when they are no longer in school, they have another outlet that’s pouring into them and they’re not getting involved in things that can be harmful to themselves and others when they are outside of the school walls,” she said.
If the program is successful, Rasoul — who serves as the chair of the House Education Committee — said he hopes it will expand to other schools and grade levels.
“This is a great way to keep students focused, especially through the summer, and to build some healthy habits with a very specific curriculum that then follows them throughout their eighth grade year,” said Rasoul.
According to the pilot program legislation, the initiative will provide community engagement, workforce development, postsecondary education exploration, social-emotional education and development opportunities to students during the academic year after regular school hours and during the summer months.
Schools will collect data and report the program’s progress to the governor’s administration and General Assembly every November for the next two years.
Public interest in youth gun violence prevention has increased, most notably after a then-six-year-old student brought a firearm from home to his Newport News elementary school last year and shot his teacher. The teacher, Abigail Zwerner, was seriously injured but survived.
The Community Builders program might have scored a legislative win, but funding for the program will remain unclear until the governor and leaders from the General Assembly determine the final budget before the June 30 deadline.
Virginia legislature will consider reworked state budget in May 13 special session
The General Assembly backed the pilot program with $800,000 in dedicated funds over the next two years. However, the governor amended the budget, cutting the request to $400,000. It’s an example of the governor’s and the General Assembly’s differing opinions on how the commonwealth should be funded for the next two years.
Del. Mike Cherry, R-Colonial Heights, who supported the Community Builders legislation, said during the Jan. 30 House Education subcommittee hearing that he believes it to be a “great model program” and would work well with Ceasefire Virginia in supporting communities facing high levels of crime.
In 2022, Ceasefire was launched as a multi-jurisdictional approach to address violent criminal activity among serious and repeat offenders in partnership with Virginia’s attorney general’s office, elected officials and law enforcement.
The purpose of the initiative is to reduce violent crime through partnerships and investments into gang prevention and community policing. Ceasefire has been implemented in 13 cities statewide, including Petersburg and Roanoke.
“When you ask high school students ‘When did things start to go wrong?’ many times they will point to the middle school level,” Verletta White, superintendent of Roanoke City Public Schools, said during a Jan. 30 House Education subcommittee hearing.
“We want to target our rising eighth graders and show them not only the detrimental effects of violence on a community, but their responsibility and how they can be community builders instead.”
by Nathaniel Cline, Virginia Mercury
Virginia Mercury is part of States Newsroom, a nonprofit news network supported by grants and a coalition of donors as a 501c(3) public charity. Virginia Mercury maintains editorial independence. Contact Editor Samantha Willis for questions: info@virginiamercury.com. Follow Virginia Mercury on Facebook and Twitter.
State News
Virginia Legislature Will Consider Reworked State Budget in May 13 Special Session
Gov. Glenn Youngkin and lawmakers have agreed to work together on the biennium budget, after clashing for weeks over two distinctly different spending plans.
A special session will be held on May 13, Youngkin and lawmakers in both chambers announced Wednesday, to consider the revamped budget and prevent a shutdown ahead of July 1, when the current budget expires.


Gov. Glenn Youngkin was joined by Democratic and Republican leaders from both chambers in the Capitol’s rotunda on April 17. (Nathaniel Cline/Virginia Mercury)
On Wednesday, the House of Delegates voted to reject all 233 of the governor’s amendments to the budget, and agreed to seek a new budget to present to the legislature May 13, with voting on it expected May 15. They also took up the governor’s other bill amendments and 153 vetoes.
The House accepted all Youngkin’s vetoes, including bills that would have raised the minimum wage, created a Prescription Drug Affordability Board to cap drug prices, protected people who come to Virginia for reproductive health care from extradition and prohibited assault firearms in public places.
Future of skill games in Virginia still unclear as Senate rejects Youngkin’s proposal
The bill amendments up for debate included: changes to legislation that would legalize skill machines, which was rejected by the Senate; a measure that would lower the amounts Dominion Energy and Appalachian Power Company can recover from customers for their pre-construction costs of a small modular reactor, which was adopted in their respective chambers; and another that would require school boards to notify gun-owning parents annually of their responsibility to safely store firearms to keep them away from their children, which was also rejected by the delegates.
It’s not clear what will happen to the language the legislature included in its budget that would’ve ordered the state to rejoin the carbon market known as the Regional Greenhouse Gas Initiative, or RGGI, that incentivizes electricity producers to emit less carbon by making them purchase allowances to do so.
Youngkin — who passed a regulation that withdrew Virginia from RGGI despite RGGI supporters saying a legislative change was needed — has opposed participation in RGGI, while calling the fee for the allowances that utilities can recover from ratepayers a “hidden tax.” The regulation withdrawal is being challenged in court.
The budget delay also creates uncertainty for local governments trying to estimate how much funding schools will receive and the Washington Metropolitan Area Transit Authority, or Metro, which is seeking additional funding from the state to bridge its $750 million shortfall.
Before Wednesday’s veto session, the governor tried compromising on the budget with lawmakers by removing all tax increases they had approved — including the digital service sales tax he initially proposed — but also dropping the tax cuts he requested in December.
In the Capitol’s rotunda with Democratic and Republican leaders from both chambers Wednesday afternoon, Youngkin said all parties are close to a budget agreement after meeting over the last few days.
“We believe this is a good path forward for the commonwealth,”Youngkin told reporters. “It reflects the work that has been done from the General Assembly and from the governor’s office.”
He added that no decisions have been made yet on the specifics of the budget, including tax increases, but he looks forward to meeting with leaders.
“This was a collective decision, and you will see from the vote this morning that it is unanimous amongst all of us to press forward in this fashion,” Youngkin said.
House Appropriations Committee Chair Luke Torian, D-Prince William, added, “We agreed that there is nothing that’s off the table. Everything will be up for discussion and deliberations. No decisions have been made at this point.”
Senate Finance and Appropriations Committee Chair Louise Lucas, D-Portsmouth, told a reporter that they were “absolutely correct” that envisioning the governor, Democrats and Republicans standing together in the rotunda two months ago was unlikely to happen when there were different budget priorities on both sides, including Youngkin’s arena proposal to bring two professional sports teams to Northern Virginia and the Democratic-controlled legislature’s plan to raise the minimum wage and allow retail cannabis sales in the state.
“But I think what’s changed is that there has been a lot of collaboration,” Lucas said. ”I think nothing helps the process more than everybody getting together, sitting around the table and talking about what we can all do to help Virginia. I think we all had different ways we thought we were going to get there, but I think now we are going to work together towards something that will keep the temperature down a little bit.”
Sen. Ryan McDougle, R-Hanover, who, along with Lucas, met with the governor earlier this week, said he is optimistic about the process moving forward.
“That’s how you come to a resolution,” McDougle said. “Everybody’s got to come to the table and talk and be heard and once you do that you can find solutions.”
by Nathaniel Cline, Virginia Mercury
Virginia Mercury is part of States Newsroom, a nonprofit news network supported by grants and a coalition of donors as a 501c(3) public charity. Virginia Mercury maintains editorial independence. Contact Editor Samantha Willis for questions: info@virginiamercury.com. Follow Virginia Mercury on Facebook and Twitter.